Ashiana, Taylor and Raffi
April 17th 2019
The Effect that Temperature has on Glucose Concentration
Introduction: The purpose of this experiment is to better comprehend the effect of temperature on enzyme reaction rate. It is also to come to the understanding that lactose is a disaccharide made up of one glucose and one galactose. When the temperature of the substrate is increased, the lactase drops will act as a catalyst at a faster rate and there will be more glucose measured by the test strips. This is because when the temperature of substrate is increased, the reaction rate speeds up. Heat is a form of energy, when the energy is increased the particles move faster increasing the rate in which they bump into each other. Hypothesis: If the temperature of the substrate goes up, then their will be a higher glucose concentration.
Materials:
- 1 2% cream box (we will provide)
- Lactase drops
- 6 Glucose test strips
- 6 Test tubes
- 1 Test tube Rack
- 1 Kettle
- 250mL graduated cylinder
- Water
- Ice
- 250 mL Beaker
- Thermometer
Procedure:
- Measure and pour 15mL creme in the 6 test tubes on the test tube rack.
- Fill up the beaker with water and 15 ice cubes. When the water reaches below 0, measure 15mL into the graduated cylinder and pour it into the 1st test tube on the rack that holds the creme. The mixture should reach 5 degrees celsius right before the glucose strip is inserted.
- Mix in 5 lactase drops.
- Wait 15 minutes, check the that the mixture has reached the desired temperature, insert glucose test strips.
- Wait until the beaker with the water has warmed up to around 5 degrees celsius. Measure 15mL into the graduated cylinder, pour it into the 2nd test tube on the rack that holds the creme. The mixture should reach 10 degrees celsius right before the glucose strip is inserted.
- Repeat steps 3-4.
- Wait until the beaker with the water has warmed up to around 8 degrees celsius. Measure 15mL into the graduated cylinder, pour it into the 3rd test tube on the rack that holds the creme. The mixture should reach 15 degrees celsius right before the glucose strip is inserted.
- Repeat steps 3-4.
- Empty the beaker and dry it out.
- Pour room temperature tap water into the beaker. Measure 15mL into the graduated cylinder and pour it into the 4th test tube on the rack that holds the creme. The mixture should reach 20 degrees celsius right before the glucose strip is inserted.
- Repeat steps 3-4.
- Empty the beaker and dry it out.
- Boil water in the kettle, pour it into the beaker and let it cool down to around 40 degrees. Measure 15mL into the graduated cylinder and pour it into the last test tube on the rack that holds the creme. The mixture should reach 30 degrees celsius right before the glucose strip is inserted.
- Repeat steps 3-4.
- Because the water in the beaker has been sitting, it should have cooled to 35 degrees. Measure 15mL into the graduated cylinder and pour it into the 5th test tube on the rack that holds the creme. The mixture should reach 25 degrees celsius right before the glucose strip is inserted.
Note that sometimes trial and error is needed to receive the desired variables.
The independent variable in this experiment was the temperature that the creme was. The dependant variable was the concentration of glucose that was resulted from the temperature. The controls in this experiment were the amount of substrate (creme) used, the concentration of the enzyme (5 drops of the lactase drops) the type of creme used and the amount of water per test tube.
Results:
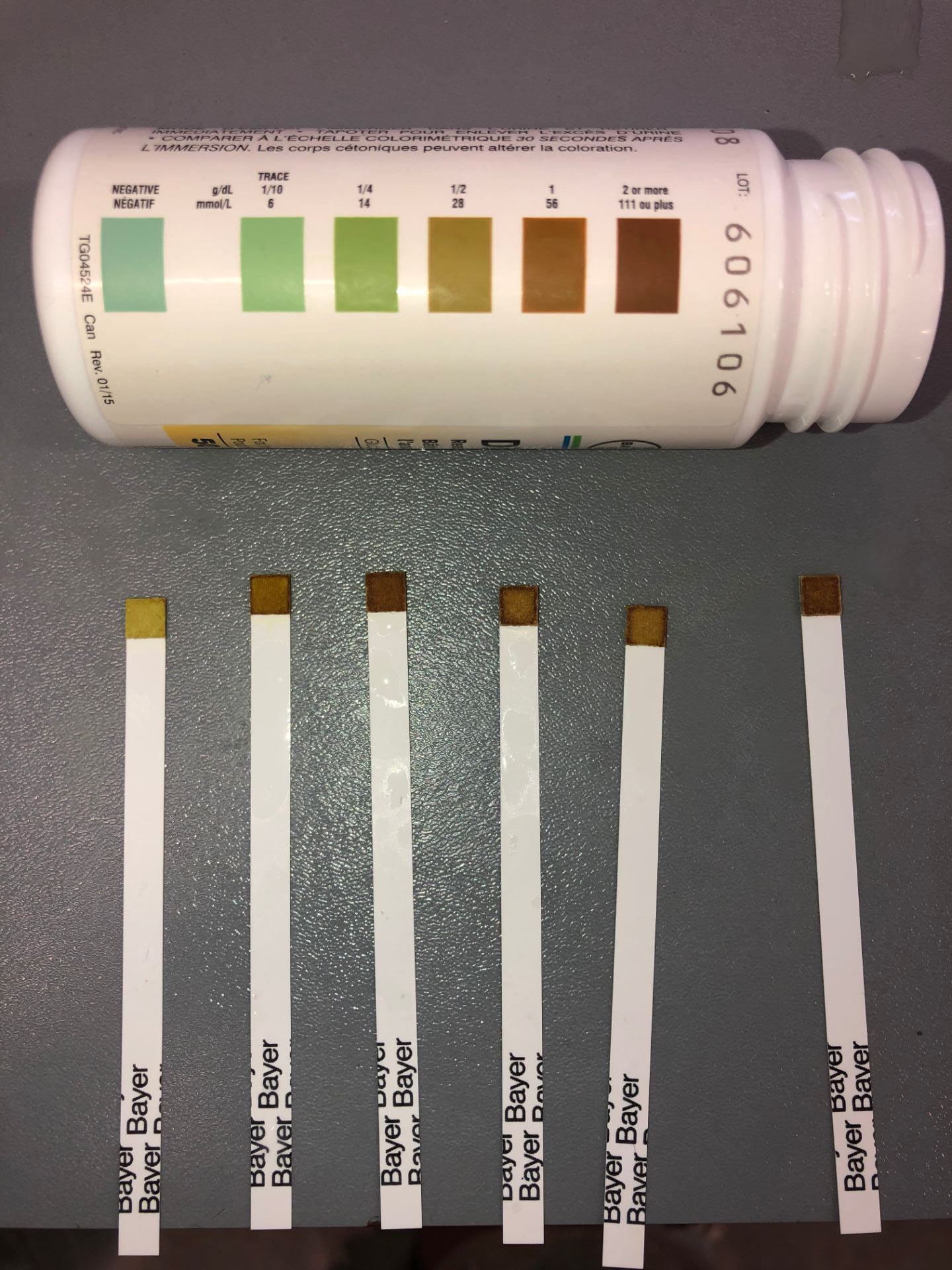
| Test Tube # | Lactose/Creme (mL) | Initial Glucose (mmol/L) | Drops of Lactase | Temperature (°C) | Colour of Strip | Final Glucose
(mmol/L) |
| 1 | 15mL | Negative | 5 | 5 °C | Dark Yellow | 28 |
| 2 | 15mL | Negative | 5 | 10 °C | Light Brown | 56 |
| 3 | 15mL | Negative | 5 | 15 °C | Dark Brown | 111 |
| 4 | 15mL | Negative | 5 | 20 °C | Light Brown | 56 |
| 5 | 15mL | Negative | 5 | 25 °C | Light Brown | 56 |
| 6 | 15mL | Negative | 5 | 30 °C | Dark Brown | 111 |
Data Analysis: It is interesting to compare the lowest temperature and the highest temperature because it followed the hypothesis and clearly differed in the amount of glucose formed. The reaction happened a lot more when the creme was 30 degrees in comparison to when it was 5 degrees. However, the middle temperatures from 10-25 all had the same amount of glucose concentration, which still follow the hypothesis because they were in between the high and low. Except for when it was 15 degrees, the glucose concentration was unexplainably a lot higher. If you look at the photo you can see how the 20 and 25 degree corresponding glucose test strips have darker edges around their light brown squares in comparison to the 10 degree which also follows the hypothesis. This overall demonstrates an increase in glucose concentration with the addition of heat.
Conclusion: In conclusion, overall the hypothesis was confirmed as their was generally an increase in glucose concentration with the addition of heat. This confirms that with the increase in temperature their is an increase in energy in the substrate that allows the enzymes and substrate to bump into each other more often, therefore increasing the rate of the reaction. Because their was more glucose when the temperature of the substrate was 30 degrees in comparison to when the temperature was 5 degrees, one can infer that lactose decomposes into glucose and galactose more efficiently with the addition of heat. When the substrate was 15 degrees there was clearly a mistake made as the hypothesis was refuted only at that specific temperature and therefore the experiment should be done again to further confirm the hypothesis.
Errors/Improvements: It was very difficult to maintain a specific temperature after adding lactase drops and waiting 15 minutes to put the glucose test strips in. The temperature continuously fluctuated and trial and error was done in adding different temperatures of water in after the 15mL of water initially added. This definitely had an effect on the results. When the substrate was 15 degrees, because it was the closest to room temperature with the addition of creme, there was no need for the addition of water. Therefore, the proportion of substrate to enzyme was more even it is possible that it increased the reaction rate. Because when the substrate was 20 degrees it required the input of warm water to keep up the temperature, there was more substrate in comparison to the enzyme so enzyme concentration was less. In order to improve this experiment there will need to be refinements made in the experiment. It will be more beneficial to have full knowledge on how fast the temperature will fluctuate so that it can be timed out that it will reach the exact temperature wanted in the 15 minutes that the enzyme needs to mix with the substrate, prior to adding the glucose test strips. The procedure will differ for every environment that the procedure is executed in, in order to maintain temperatures.
The fact that the amounts of substrate varied in each test tube is a huge error as it will effect the enzyme concentration which was supposed to be a control group. However, this was required in the experiment to maintain the temperatures desired.