Results
We tested three different fruits and vegetables by sticking a nail and copper into a fruit or vegetable. We then connected wires from zinc and copper, to a voltmeter so we could measure the voltage that the fruit and vegetables had. We set the voltmeter at three because we knew that the fruit and vegetables wouldn’t have a high amount of voltage. After we recorded the amount of voltage each fruit or vegetable had, on a chart to record their results.
The first thing we tested was an orange the orange had a voltage of 0.3
The next thing we tested was a potato that had a voltage of 0.2
The last thing that we tested was a lemon and that had a voltage of 0.7
My results Chart
The lemon: This was our highest voltage
The Orange: This was our second highest voltage
The Potato: This was our third highest charge
Here is a picture of a group that was able to get their fruit to light a small light bulb
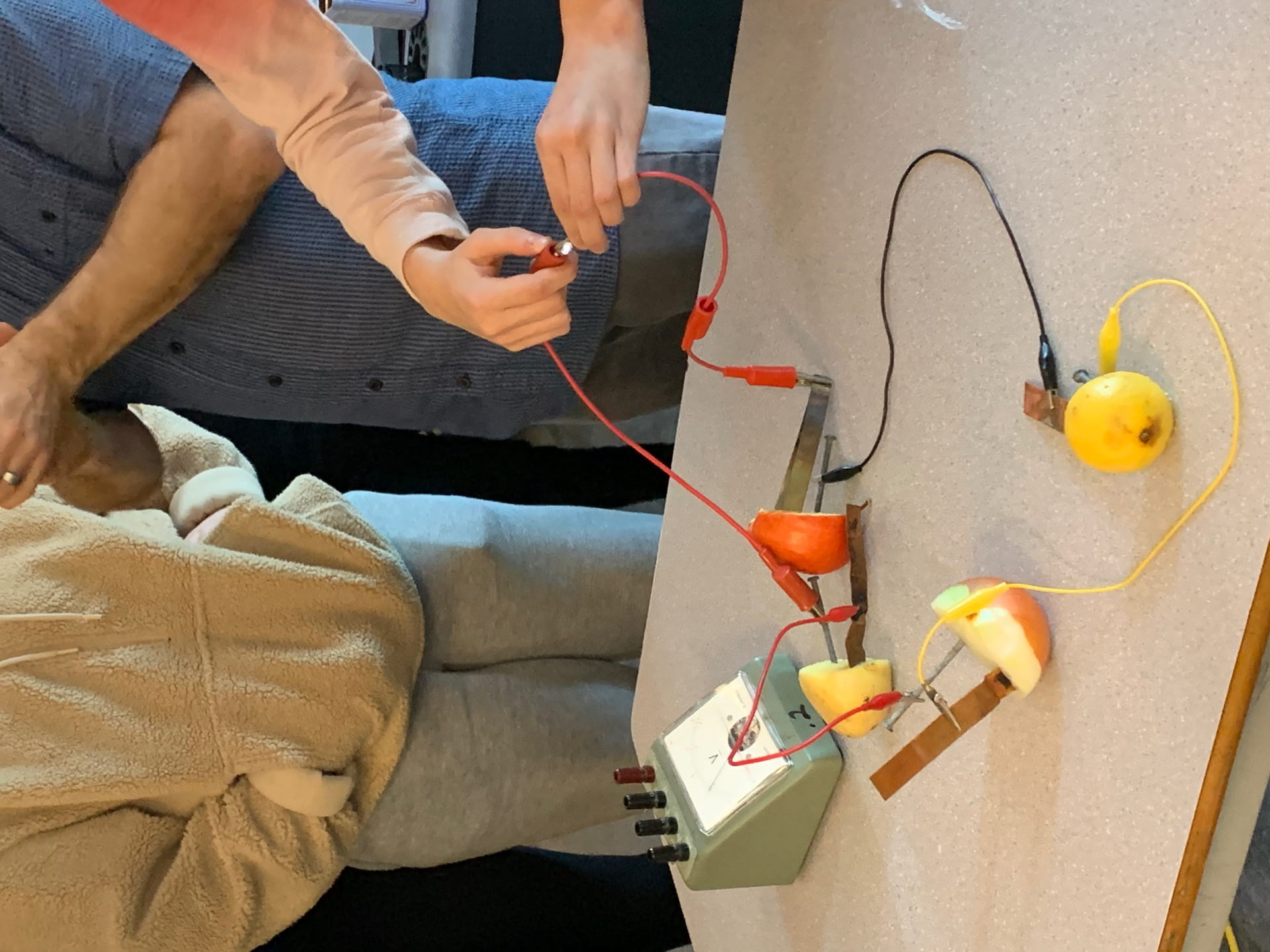
To get a light to light up they had to make sure that it was alternating zinc, copper, zinc, copper,… They also needed to get more wires and more fruit.
Observations
I noticed that it was difficult to poke the copper stick into the fruit or vegetable because it would bend but the nail did not bend
I noticed that when we poked the nail and copper into the fruits with juices some of the juice would squirt out
I noticed that the experiment worked with half of a fruit or vegetable as well as a whole fruit or vegetable would work.
Explanation
I think that the lemon had the most voltage because it is citric. From this lab I assume that if a fruit or vegetable is citrus it will create more voltage. I assume this because the orange has more voltage than the potato, but the lemon had a lot more than both the potato and the orange. I think that the acidness caused a negative charge so therefore the electrons repelled and that is why the electrons were flowing.
Prompting Questions
1.If your bulb doesn’t glow why not?
My group was unable to get our bulb to glow, but we later found out that was because we were not alternating zinc and copper. We were also not using enough wires to complete our circuit.
2. How could we modify our experiment to improve our results?
We could modify this by trying more of a variety of fruits and vegetables and trying a whole fruit to see if we can get more voltage than we got with just half of a fruit.
3.What is causing electrons to flow in this experiment?
The acidness from the lemon and the Ion charges that come from the lemon’s acidness causes the electrons to flow because opposites attract and like repel so that is what is causing the electrons to flow.




Great work testing so many fruits, sharing your observations and answering the questions. What could you do to increase the current even more?