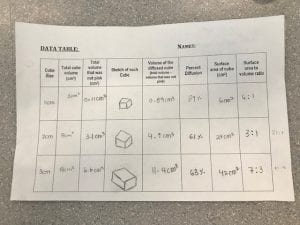
Purpose: What determines the efficiency of diffusion throughout the model “cells”?
Hypothesis: The smaller the surface area and volume, the higher the rate of diffusion will be.
1. In terms of maximizing diffusion, what was the most effective size cube that you tested?
In terms of maximizing diffusion, the most effective cube that was tested was the small, 1cm cube of agar.
2. Why was that size most effective at maximizing diffusion? What are the important factors that affect how materials diffuse into cells or tissues?
This size was most effective at maximizing diffusion because its surface area is proportionally larger than its volume, which can be seen through the surface area to volume ratio, 6:1. The smaller surface area and volume, the easier it is for diffusion to occur. Some factors that affect diffusion are: temperature, surface area, volume, and the size/shape of the molecule.
3. If a large surface area is helpful to cells, why do cells not grow to be very large?
A large surface area is helpful to cells as it allows for more materials to enter the cell, however, a smaller surface area is also helpful as it allows for diffusion to occur at a faster rate, and move towards the center of the cell faster. When a cell grows its volume increases at a greater rate than its surface area, causing diffusion to happen at a slower rate, thus, decreasing growth.
4. You have three cubes; A, B, and C. They have surface to volume ratios of 3:1, 5:2, and 4:1 respectively. Which of these cubes is going to be the most effective at maximizing diffusion? How do you know this?
Cube C would be the most effective at maximizing diffusion. This is due to the fact that the surface area to volume ratio (4:1) contains a surface area that is proportionately larger than the volume. There is more surface area to be diffused but, the smaller volume allows for diffusion to travel and reach the center of the cell quicker.
5. How does your body adapt surface area-to-volume ratios to help exchange gases?
Our lungs contain sacs known as Alveoli. These sacs have a large surface area to volume ratio due to their folded shape, causing diffusion to happen more quickly. Each sac contains a thin wall which increases their surface area and makes it easier for them to deal with any inflation that may occur in the lungs. This also decrease the amount of volume that diffusion needs to act upon.
6. Why can’t certain cells, like bacteria, get to be the size of a small fish?
Certain cells can’t get to the size of a small fish as it wouldn’t be practical for them. It will make it harder for cells to diffuse materials that well. The cells are small to begin with as it allows for diffusion to occur at a much greater rate. Increasing the size of the cells would only slow down the rate of diffusion for the cell.
7. What are the advantages of large organisms being multicellular?
Some of the advantages of large organisms being multicellular is that they grow. Single celled organisms can only reproduce and make more copies of themselves, they are not capable of growing. Another advantage is that due to them being multicellular, they can have multiple functions, making them more efficient.