Category: Science 9
How Things Work – Solar Panels
Solar Panels
What are solar panels used for? Solar panels are used to try to solve the problem of using renewable energy instead of non-renewable. They also try to make electricity cheaper which they do by reducing electricity bills for people in sunny areas. People in places like California, Arizona etc. can save money by using solar panels to power their homes.
How do solar panels work and what makes them work? These are the most important parts. The most noticeable part of a solar panel is the solar panel itself. The solar panels are usually placed on the roofs of houses although they can also be placed in large fields with other solar panels or on free-standing poles. The solar panel is what converts the sunlight into electricity. These panels are placed on solar array mounting racks. The racks hold up the solar panels and are angled so that they can use the sun as much as possible. There are two types of mounting racks. The cheaper kind, fixed mounts, have a fixed height and angle for maximum efficiency, but the sun moves throughout the day and throughout the year, so your solar panels won’t always be working to the best of their abilities. The more expansive rack is called a tracking array. Tracking arrays move east to west with the sun and adjust their angle to use the most sun possible. These solar panels need maintenance and you can’t just take them apart while they are on. The array DC disconnect is used to disconnect the solar panels from the house while doing maintenance. The reason it is called the DC disconnect is because the electricity that solar panels produce is direct current. Standard appliances in most peoples homes use AC. That’s why you need an inverter. Inverters convert the DC from the solar panels, to AC so that it works with your appliances. Solar panels don’t work all the time because days can be cloudy, and there is night. That’s why most solar panels have batteries. The excess electricity made during the sunny hours, go into a battery pack that holds the electricity until its needed. Although, the batteries can’t hold too much voltage. A charge controller fixes that problem. The charge controller allows you to maintain the proper amount of charge going into your battery, and when the electricity comes out of your battery into your appliances. The electricity enters your home at the breaker panel. There is a circuit breaker that prevents the appliances on a circuit from drawing too much electricity and causing a fire hazard. If this happens, the circuit breaker will switch off, interrupting the flow of electricity. These are the main parts of a solar panel that do the most to keep your system running.
How do solar panels actually work? The answer is chemistry. The main element in solar panels is silicon. Silicon atoms have room for eight electrons in their valence shell but naturally carry only four. Silicon bonds strongly together so this is what makes the plates of the solar panels because the silicon allows an easy platform for electrons to flow. Silicon alone does not create electricity so it is chemically combined with phosphorous, and chemically combined with boron. Phosphorous has five electrons that it gives so when combined with silicon, the result is negatively charged. Boron only has three electrons to offer so the result is positive. Sunlight sends many different particles of energy but the one used by solar panels is called photon. If the panels are faced right, the photon from the sun’s rays, hits the silicon-phosphorous mix and eventually knocks the extra electron out of its shell. One of these doesn’t create much energy, but together they start to build up. These negative electrons are attracted to the positive phosphorous-boron mix (because opposites attract) which is attached to wires. The electrons then move through the wires that connect to the home or whatever is getting charged.
Solar panels have many strengths, but also many weaknesses. One of the biggest strengths is that it gives a different option for a more renewable power supply. Instead of having to burn fossil fuels, and create nuclear waste, solar panels makes for a completely eco-friendly energy source. Even Hydro dams have a problem with not letting fish get up rivers. Solar panels use nothing but the sun to make energy. Their next biggest strength is cost effectiveness. Solar panels can cost a lot to install, however, if you live in a sunny area the price can actually be paid off and eventually, you can save money. Solar panels take a lot of money off energy bills so if you live somewhere sunny, the money you save from energy bills will eventually pay back the money spent on buying and installing the solar panels. These are the biggest strengths but, solar panels also have some pretty big weaknesses.
The main two weaknesses are connected; they can’t run all day because there isn’t any sun at night, and they don’t work very well in places that don’t have as much sun, for example, right here in British Columbia. With solar panels, you still need normal electricity, because it doesn’t last all day, so in the hours with no sun, you need an alternative. In places without enough sun, its just not worth it because you’ll end up paying more for installing it and paying for the solar panels, than you would save from actually using them. Yes solar panels can help, and yes, they are eco-friendly, but they only work in the right conditions, so its not a complete solution to the problem it is trying to solve.
The problem solar panels are trying to fix is using more renewable energy. Do solar panels use renewable energy? Absolutely, but do solar panels fix the problem entirely? Although solar panels are useful, and a good start to the fight against non-renewable energy, they don’t fix the problem completely. People in sunny areas might be able to get by with them if each day is perfect, but if not, they will usually have to rely on a secondary source of power. Solar panels are useful, and they definitely eliminate the use of some non-renewables, however, they don’t quite fix their problem.
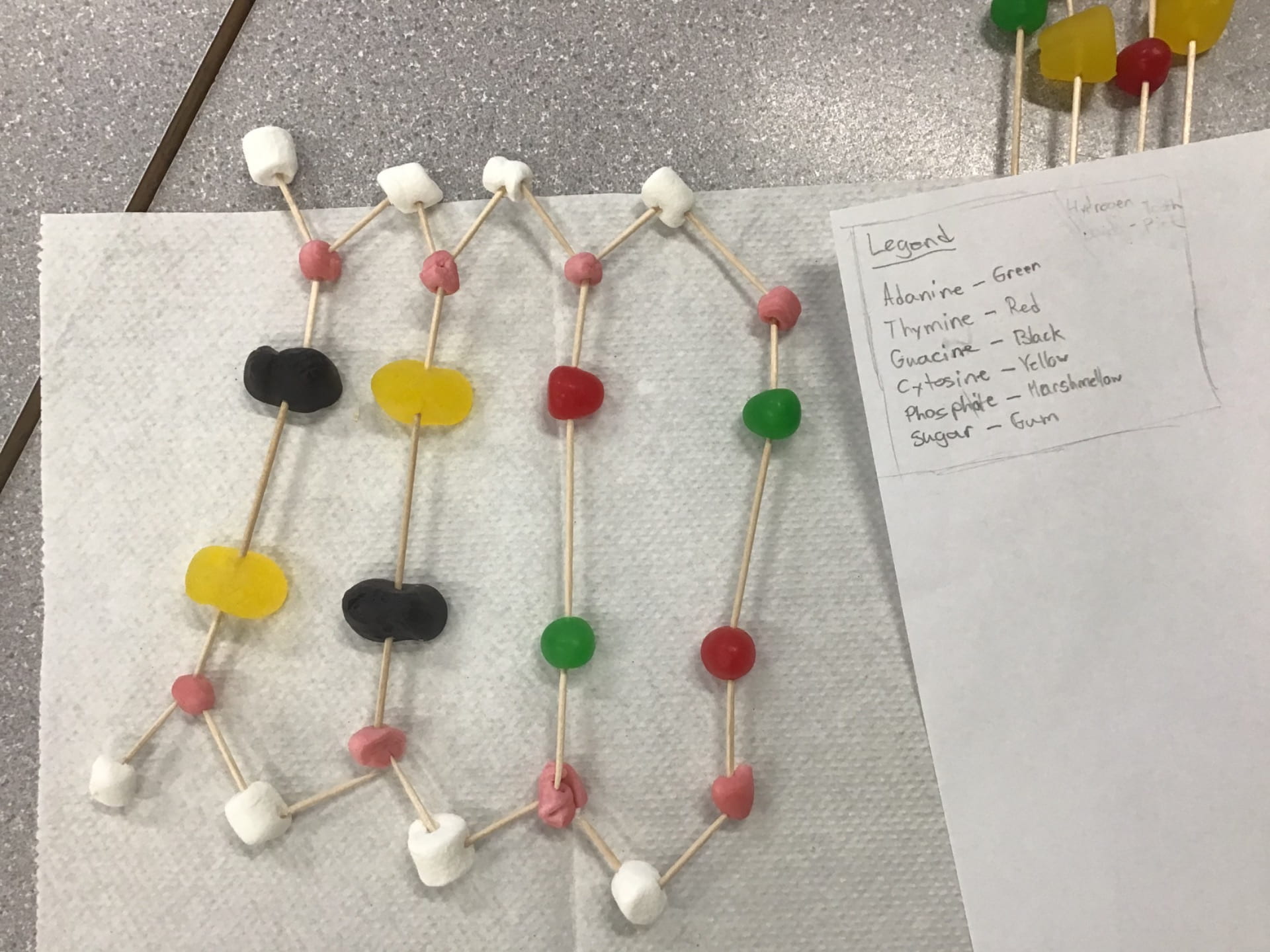
Model
Sources
“Components of A Residential Solar Electric System.” Cleanenergyauthority.com, Chris, 6 Jan. 2010, https://www.cleanenergyauthority.com/solar-energy-resources/components-of-a-residential-solar-electric-system.
Pollick, Michael, and Niki Foster. “How Do Solar Panels Work?” WiseGEEK, Conjecture Corporation, 29 Nov. 2019, https://www.wisegeek.com/how-do-solar-panels-work.htm.
Fox, Stuart. “Sizing the DC Disconnect for Solar PV Systems.” CivicSolar, Inc., 11 Nov. 2011, https://www.civicsolar.com/article/sizing-dc-disconnect-solar-pv-systems.
“Tubular sunshine; Solar energy.” The Economist, 11 Oct. 2008, p. 99EU. Gale In Context: Canada, https://link.gale.com/apps/doc/A186568185/GPS?u=43sbo&sid=GPS&xid=fe04895e. Accessed 11 Dec. 2019.
A Fresh Look at the Periodic Table
My Periodic Table of Elements Explanation
Me and my partner’s periodic table is sorted in a number of different ways for a different view on the elements and for easier use when exploring the ionic charges of elements. Our periodic table has five patterns that help the user identify elements certain qualities. Our periodic table’s main pattern is that it is arranged by ionic charge. Each of the twelve sections hold elements with the corresponding ionic charge labeled in that section. There is positive one through seven, negative one to three, an other section for the elements that have ionic charges that are not shown because not enough is known about them or they don’t have a predictable ionic charge, and finally, a neutral section for the noble gases. In each of the sections, elements are arranged by atomic number so the element is easier to find in each section. The solids, liquids and gases are all sorted by colour. All of the solids are white, while the liquids are shaded blue, and the gases are shaded yellow. We kept this trait from the original periodic table because without it, one would not know whether an object was a solid, liquid, or gas; they would either have to know from prior knowledge, or research to find out. The next organizational method we used was also from the original periodic table. We have a red box separating our non-metals from our metals so that the user can easily figure out if an element is a metal or not. The last thing we did to categorize the elements on our periodic table was keeping the different families together. We left the transitioning metals white, but the other families we highlighted. Elements with a purple highlighted symbol and star belong to the alkali metal family. The alkaline earth metals have a green highlighted symbol and green square. An orange triangle and highlighted symbol, means they are part of the halogen family. Lastly, the noble gases have a pink highlighted symbol and contain a pink circle. With these different qualities, our periodic table allows easy use, showing the user details such as the element being a non-metal or metal, solid, liquid, or gas, and what family each element exist in. With this, and being sorted by ionic charge, our periodic table is a useful variation of the original that offers its own benefits that the original did not have.
New Periodic Table Project (6 D’s)
Define and Discover:
- Re-organize/re-create the periodic table of elements to make it more creative, more efficient, more visually appealing, or more effective.
- How could you make it more visually appealing without decreasing its effectiveness?
- What should be kept the same?
- What variations have already been created?
Dream:
- The periodic table tells us the amount of protons, neutrons, and electrons. As well as the atomic mass and how many elements there are. Also which elements are metals, non-metals, solids, liquids, and gases, and the ionic charge(s) of each.
- We could arrange it by state (solid, liquid, gas). Switch the families and periods around. Most common to least common (rarity). Ionic charge. Amount of neutrons or electrons. Electron shells. Chemical properties such as reactivity, magnetism, etc.
- We could use shapes for non-metals and different shapes for metals. Do the same except for solids, liquids, and gases. Shape it all into a circle or other shape. Shape it into a picture, for ex. A Bohr diagram. Different shapes for synthetics
- Have each family a different colour, arrange by colour of element, colour each period, colour by ionic charge, colour by atomic mass.
Design:
- Our plan is to organize each element into groups and see how we can keep them together once we order them on the periodic table.
- Use trial and error to look at each of our options and see which one is best.
- Once we have figured out what we are going to do, waste no time creating it, adding in things as we see fit.
Deliver:
- See below our periodic table for the explanation of it.
Debrief:
- Me and my partner worked well together to create a well thought out variation of the periodic table. However, we could’ve done a couple of things better. The first improvement is that we should’ve came to a decision on how to organize our periodic table quicker. We took too much time trying to think of what way we would organize the periodic table. We should’ve made our decision on that faster so that we had more time to work on everything else. The second thing we could’ve done better is making a good copy of our periodic table. Our variation of the periodic table looks a little rough because we didn’t have enough time to make a better copy. We probably could have done it if we hadn’t spent so much time deciding how we would organize our periodic table. Despite this, I think we did a very good job and created a useful variation of a periodic table that gives a new look on the elements in a different order.
Mystery Powder Lab
Mystery Powder Lab Questions
- Explain the difference between a chemical and physical change.
A chemical change is a change in matter that happens when substances combine to create new substances that were not there before. A chemical change can usually be identified by a the substance having new colour, giving off light or heat that wasn’t there, the forming of bubbles or gas, solids changing to a liquid, or by testing how hard the change is to reverse. A physical change is a change in appearance but unlike the chemical change, no new substances are formed. For example, ice melting is a physical change because even though a solid became a liquid, no new substances formed.
- For unknown D, explain the results of each test, including what you saw and whether what you saw was a chemical or physical change.
Me and my group did six different tests on seven powders. We did a test on appearance, whether it dissolved in water, what happened when heated, and what happened when it came into contact with universal indicator, an iodine solution, and vinegar. For unknown powder D the appearance was powdery with small clumps, it was a dirty white colour and was matte. It stuck to walls like unknown C but not quite as much. The next test we conducted was whether or not the powders dissolved in water. Unknown D did not although it became thicker and pasty. The change here was physical because no know substances were created. The third test was what happened to the powder when heated. Powder D stayed the same as did the rest of the powders except for E and Z. The change in powder D was neither physical nor chemical as there was no change. We then tested what would happen when it came into contact with universal indicator. When the eye dropper’s drops of universal indicator hit powder D, it solidified and turned yellow which is a chemical change because a new substance formed. We know this because a liquid turned into a solid with no heat taken away and no pressure added. The next test was what happened when the powders came into contact with the iodine solution. When the iodine hit unknown powder D, the iodine turned brown and stayed a liquid although it didn’t mix in with the powder. This was a physical change as iodine is already a shade of brown so not much changed. The last test we performed on the unknown powders was what happened to them when they came in contact with vinegar. The vinegar-powder mix became sticky where the vinegar hit unknown D and the vinegar became a solid. This change was chemical as the liquid vinegar became a solid without change in temperature or pressure and it became a sticky substance which was not there before.
- Based on your results identify which two powders make up Mystery Powder X and which two powders make up Mystery Powder Z?
Based on our test results, we think that unknown powders C and E made up mystery X, and that A and D made mystery Z. C and E make X because of certain changes in each of them that only happened in either C and X or E and X. For example, when C came into contact with the universal indicator it solidified. When E did, it turned green and X solidified and turned green. Also when the vinegar was dropped onto the powders, the only two that bubbled up were E and X, although X not as much which makes sense because it must have been diluted with C. This is why X is made up of C and E. As for Z there are only three left. We know that one of them is powder D because Z is powdery and the only two that are powdery are C and D which C has already been used. Also D and Z are the only two powders that solidified and turned yellow when they came into contact with universal indicator. As for the other powder that makes up Z it can only be either A or B. This one was a little more difficult to decide on which one was in Z because not as many properties shared. My conclusion was that it was A in Z because of some small similarities. For example, Z is a powder but A and B are both crystals. However, A has smaller crystals and B’s crystals would probably be evident in Z. Another reason is that when tested with universal indicator, Z becomes solid and yellow like D. B stays the same but orange, and A stays the same but a yellowy-orange. If B was in Z, Z would probably not have become yellow but more of an orange. Mystery X is C and E, and mystery Z is A and D
- Explain any experimental sources of error from this lab (where could you have possibly made a mistake?)
I think me and my group did really good and there was only two mistakes I could find. The first one was that when heating, we had to look at the bottom of the cups of powder for colour because we put a little too much in and did not have time to heat the top. The second mistake was we might’ve done something wrong when testing A because I’m not sure if the results should have been that close between A and B being in Z. I could still find that A was the one in Z but if I had the time I would have re-done the testing on A and heated the powders again with a little less in them.
Grey Water Experiment Project
How well do plants grow when watered with dishwashing grey water (with Dawn dish washing soap)?
Part A: Question, Research, and Hypothesis
Research: We found out that plants, especially fruit trees can survive on grey water, so we want to find out if they help the plants grow better than with regular tap water. We found out that you shouldn’t put the grey water on edible parts of the plant because it can harm the humans eating the plants due to the dangerous bacteria and chemicals in the soap and grey water. Some well developed countries don’t trust the use of grey water but countries that suffer droughts that are getting worse as the years pass need to use grey water as they don’t have a large supply of fresh water for plants.
Hypothesis And Reasoning: Our hypothesis is that plants watered with grey water will still grow but not as much as the plants watered with tap water. We think that the bacteria and/or chemicals that we’re exposing the plants to will stunt its growth not letting it reach its full height. Some countries don’t trust grey water, but others are starting to use it, giving us more trust that our plants will still grow if not to their maximum height.
Part B: Plan
Investigation Plan: We will start by getting all our supplies and then by setting up our workspace. On the first day we will water half of our plants with tap water and half of our plants with grey water. We will see if there are any immediate effects. Every couple of days, we will check our plants and take a picture to keep track of our data. On every Monday, Wednesday, and Friday, we will water our plants. On the final day we will check our data and our plants to see if there are any significant differences between the tap water plants and the grey water plants.
Collection Of Data Materials: We will be using two materials to collect our data. The two materials are a camera on an iPad to keep track of the visual changes, and a word document to keep a small journal on day-to-day changes of the plants. These will help us in the end to come to a conclusion on whether the plants watered with grey water were affected at all.
Safety Issues And Risks: There is little to no risk in this experiment. The worst-case scenario is either getting a little dirty, or soap in your eyes; both not life-threatening safety issues.
Ethical, Cultural, And Environmental Issues: As far as what we are doing, there should be no issues, in fact some cultures would probably think its good what we are doing. Our experiment can help people that live in places that don’t have as good access to water as we do. They can look at the results of our experiment or do it for themselves to see if they can use the same water that they washed their dishes with for their plants. As for the First Nations, we can see no way that we are creating any issues with them by conducting this experiment.
Part C: Data
Plant Data Journals
Sep. 24th 2019:
The (TYPE OF PLANT) are in the classroom today with their first water. All look very similar and I would not be able to tell them apart.

Sep. 25th 2019:
After one day there is still no change which is as expected.

Sep. 27th 2019:
Another two days and still no change. All four plants, both the two tap water and the two grey water are looking healthy.

October 2nd 2019:
Finally we are starting to see a little change. The two grey water plants are starting to look slightly more dry despite the fact that we water both equally.

October 7th 2019:
Almost another week later the differences between the two are becoming more recognizable. The plants watered by tap water seem to have slightly richer colours in both leaves and stem.

October 11th 2019:
The plants watered by grey water are just becoming more noticeable by how dry they are looking. The grey water plants stems look lighter and not quite so rich in colour.
October 15th 2019:
Our last day of watering. The tap watered plants look healthier in stems and leaves than the grey water plants. Even the height is slightly greater.

October 16th 2019:
We start making our graph and analyzing our data. We have one last look at the plants before we start to write our results. The tap water plants look much more healthy than the grey water plants. Their leaves are greener, there stems are deeper in colour, and they are slightly taller. The biggest difference between the two is that the grey water’s plants leaves look a lot more dry and some even look a little shrivelled with small blemishes on them.
Graphs:
Part D: Analyze
Height Graph: The height of the plant can determine how healthy the plant is by how much it has grown. Healthier plants should be taller as they grow to their full height. The tap water plants are both taller than the grey water plants although not by much which is to be expected as they have only been growing under grey water for three weeks. The plant colour however is a lot more noticeable despite the short time when watered with grey water.
Plant Colour Graph: We can determine how healthy each plant is or how dry each plant is be looking at the colour of the leaves. The grey water plants leaves are a lot lighter shade of green and look like they need a little more water despite the fact that they have been watered equally throughout the three weeks.
Relationship Between Variables: Our variable that we changed for each of the two sets of plants was what we watered each with. The first two plants we watered with ordinary tap water. The second two plants we watered with grey water (Dish washing soap and water with Dawn dish soap). The relationship between the plants watered with grey water and the plants watered with tap water is that the grey water plants look more dry and don’t have as deep colours. They are also slightly smaller and have some small blemishes on the leaves. This shows that when watered with soapy water, the plants become less healthy than when watered with tap water.
Inconsistencies: The only small inconsistency was that one of the tap water plants also had a couple of small blemishes on the leaves but they were minor compared to the ones on the leaves of the grey water plants.
Part E: Conclusion
Hypothesis (was it supported), Our Conclusion, And Can There Be Other Conclusions: The data that we collected supported our hypothesis. Our hypothesis is, plants watered with grey water will still grow but not as much as the plants watered with tap water. We were correct to state this although there wasn’t as big a difference in height as we thought there would be. However, the plants watered with grey water differed from the tap water plants in another way. The grey water plants look more dry than the tap water plants even though we watered each equally. Both leaves and stems of the grey water plants didn’t look quite as healthy and that brings us to our conclusion. Over the span of three weeks, we have determined that a tropical indoor plant will grow when watered with grey water. However, it will not grow as well as one watered with tap water as they may not reach their full height and become dry even if watered regularly. Our conclusion should be the only conclusion even if others do the same experiment. The only way somebody doing our experiments conclusion could change is if the plants don’t get the exact same treatment (except for the variable) or if different plants are used.
Methods And Conditions Of Experiment:
Sources of Error: We did pretty good in not having anything wrong but even the best scientists make mistakes. Our biggest mistake was not measuring the height of the plants at the start of our project so even though we know that the tap water plants are bigger, we don’t know exactly how much each one grew.
Confusing Variables: Throughout the three weeks, we did everything exactly the same to each of the four plants. The only variable that we changed was that two of them we watered with tap water, while the other two we watered with grey water (water with mixed in Dawn dish washing soap). We found that we didn’t have any confusing variables because we were so careful to make sure that everything was done the same to each plant.
Areas For Improvement: In any experiment there is always ways to improve. In our experiment we could’ve improved a couple of things such as measuring the height of our plants at the start of our experiment. We could have also used more plants as we had two for each tap and grey water. The data probably would’ve been more reliable had we used three plants for each tap and grey water. These are the two biggest ways we could’ve improved our experiment but as in all experiments there is always many different ways to improve no matter how small the improvement.
SMART Goal Reflection
1. What was your goal?
My goal was to walk home from school every day for one week
2. Did you achieve it? How or how not?
I did achieve my goal by making sure my parents knew not to pick me up and not have me do something immediately after school
3. What was the most challenging part of achieving your goal?
The hardest part was making sure that I didn’t have to go somewhere after because one of the days my dad was going to pick me up after school to get new hockey gloves so I had to rearrange that
4. What would you do differently next time?
I would have myself walk to school instead of walking back home because sometimes I have to get driven to go somewhere
5. Will you continue with this type of goal? If you do, explain what you will do. If not, explain why you are satisfied with where you are at.
I will try to continue to walk to and from school as much as possible and I’m satisfied even if I don’t do it every day because sometimes I will need to go places or get home quick for something.
Water Quality in the Coquitlam River and Oxbow Pond
Over the past week in Ms. Brandsma’s science 9 class, expert scientists Noah, Braden, and myself (We are actually just students), have been conducting experiments on the water quality of two water bodies in Port Coquitlam. The Coquitlam River and Oxbow Pond are both located within less than a kilometre away from Gates park. Despite this, one is a clean-looking fast-flowing river, and the other looks to be more of a disgusting swamp. However, looks can be deceiving. The information we took from our tests consists of temperature tests, chemical analysis’s and the presence of aquatic invertebrates. The information from these three tests will help us determine the water quality of both Oxbow Pond, and Coquitlam River.
The first test we conducted on each of the water bodies was temperature. By finding the temperature, we can find whether the water is in good condition for fish and other aquatic animals. Water temperature can help us determine whether or not the water is in good condition because water that is too warm has a higher fish disease risk and different types of fish. Colder water temperatures result in a lower risk of fish disease and the type of fish we have here. Colder water also tends to have more dissolved oxygen than warm water which helps aquatic life survive. We took three different tests in three different spots for both bodies of water to find the temperature of each. All of our tests resulted in us coming to the conclusion that both bodies of water have a colder temperature which means that temperature wise, they are quite healthy. The Coquitlam River had an average temperature of 11 degrees Celsius, and the Oxbow Pond had an average temperature of 9.6 degrees Celsius, both falling into the cold category of water temperature (5-13 degrees Celsius).
Chemical analysis’s are a big help in determining whether or not a body of water has good water quality. At both Oxbow Pond, and Coquitlam River, my friends and I performed five different chemical analysis’s. The first was a test on pH levels or how acidic the water is. Pure water has a pH level of 7. The lower the number, the more acidic, and the higher the number (up to 14), the more alkaline. Most aquatic animals prefer a pH level from 6 to 8.5. The water at both bodies of water we tested fit in between 6 and 8.5 but weren’t quite pure. Oxbow pond had a pH level of 6.5, and Coquitlam River 6.3. Therefore, both are healthy enough for aquatic animals as for as there pH level.
The second and third chemical analysis we conducted was Nitrate and Nitrite levels. Nitrates are found in water from things such as fertilizer runoff, sewage treatment plants, manure runoff, and exhaust from vehicles. Nitrates in small amounts can’t do much but Nitrites are different. Nitrites do not last long as they are usually quickly converted into Nitrates by bacteria. Nitrites can create a serious illness called brown blood disease in fish. This can also affect babies especially when under three months of age which causes a condition known as blue baby disease. The Nitrate levels were 3.3 ppm (parts per million) in the Coquitlam River. However in Oxbow Pond, the Nitrate levels were 13.3 ppm. Although the Nitrite levels were a lot closer in fact, our tests showed that both Pond and River had a Nitrite level of 0.3 ppm. As for Nitrites, Oxbow Pond and Coquitlam River have suitable levels as there is a limit of 1 ppm on drinking water and both bodies of water fit under that.
The fourth chemical analysis we did was testing the hardness of the water. The hardness of water refers to the amount of dissolved calcium and magnesium particles in the water. Hard water is safe for human consumption, however it can damage household items such as shower heads and pipes. Hard water is also safe for aquatic animals, the only part of water hardness that can harm organisms and animals is when it suddenly changes from soft to hard or the other way around. The hardness of water in the Coquitlam River and Oxbow Pond are both quite similar, Oxbow pond having 40 ppm and Coquitlam River having 33.3 ppm. Both bodies of water have soft water so we can say that both are healthy based on the tests we performed as we did not measure changes in the hardness.
The last chemical analysis we conducted was testing carbonate levels. Carbonate in water is what changes the hardness. The more carbonate in the water, the harder the water is. The Coquitlam River has a lower carbonate level than Oxbow Pond which matches its hardness. The Coquitlam River had a carbonate level of 10 ppm where Oxbow Pond had a higher level of 50 ppm which also matches its slightly harder water. Despite the levels of carbonate being higher in Oxbow Pond, it still has soft water. Both Pond and River have relatively low carbonate levels which matches their soft water, therefore the bodies of water have healthy carbonate levels.
The final test we conducted to determine the water quality of the Coquitlam River and Oxbow Pond was finding invertebrates in these bodies of water to see what types were there. This helps because different invertebrates need different conditions to survive, and are more tolerable of pollution than others. The invertebrates that we found in the Coquitlam River consisted in Mayfly nymphs (scraper), Stonefly nymphs (shredder), Water Striders, Midge larva, and Dragonfly nymph (predator). Both Stonefly nymph and Mayfly nymph are found in good quality water as they are pollution sensitive. The Dragonfly nymph is somewhat pollution tolerant. The Midge larva can be found in any quality of water. Water skimmers aren’t as good an indicator as the other invertebrates as it doesn’t depend as much on the water (lives on surface). Taking the information that these five invertebrates give us, we can say that the Coquitlam River has good water quality. Although there are organisms living in the river that are pollution tolerant, this just means that they could be in any type of water. As for the invertebrates more sensitive to pollution, they wouldn’t be found in polluted water.
The invertebrates found in Oxbow Pond were definitely different. The only one that existed in both was the Dragonfly nymph. Besides that there was also the Riffle beetle, Damselfly, and Leech. The Riffle beetle is found in good quality water as it is sensitive to pollution. The Dragonfly nymph, as said before, is somewhat pollution tolerant as well as the Damselfly. The leech can be found in any quality of water, being pollution tolerant. Despite the smaller amount of pollution tolerant invertebrates, we can still say that Oxbow Pond has good water quality as the Riffle beetle wouldn’t be able to live there if it was polluted.
Finally, to determine whether Oxbow Pond, and Coquitlam River have good water quality, we add up the each of the results from the three tests we conducted. The results show that even though Oxbow Pond might look a little like a swamp, it still has good water quality, maybe even as good as the Coquitlam River. All in all, both bodies of water have good water quality despite their different looks, and are not polluted.
Reflection: I thought this project was a fun way to look at the water quality of water bodies near where we live. My class and I got to learn how to test chemical analysis’s, locate invertebrates in the water and see what this meant for the quality of water the said tests were performed in. We got to work outside for four days and it was a project I would definitely suggest for my teacher to continue assigning and would also encourage other teachers to try it out as well.
Where would alien life most likely be if we found it?
Where would alien life most likely be if we found it?
The universe is so unimaginably huge that we don’t even know if it ends. Researchers are looking for life in the universe and to find it, they need to think where it would be? Realistically life could be anywhere, because whatever life there is out there, could’ve evolved to the conditions. The thing is, we can’t look everywhere because our technology limits us, and there is way too much out there. So how do we narrow it down. Take the most reliable ways scientists are using to find life and put them together. Some of these include searching for water, stars’ habitable zones, and oxygen and methane found together. Wherever there are planets that are found to have these traits, there is a higher probability that they have life.
The number one way (in my opinion) that scientists are looking for life, is searching for water. Every known living thing on Earth needs water. Based on this, any life that we find, should need water. Researchers have been looking at Mars a lot because of the dried up riverbeds and permafrost (ground whether its rock or soil, that remains frozen for two or more years) that they have found. However, the search for planets, have lead scientists to focus in on one of Jupiter’s moons, Europa. Europa is a moon that is pretty much a ball of very thick ice, but this ball of ice might not be so thick in one place. According to Corey S. Powell, writer of Europa or Bust, “Researchers working with the Hubble Space Telescope sighted a huge vapour cloud hovering over Europa’s southern hemisphere. Evidently liquid water is able to break through the crust and blow into space, meaning that either there is water close to the surface or there are very deep cracks in the ice.” Using these cracks or the thinner ice we could see if a probe could get down into the water to see whether there’s life. Getting through this ice would be an incredible feat and if scientists even managed to do it there might not be life. All this relates to another way researchers are looking for life.
Europa is 780 million kilometres from the sun. The suns habitable zone starts roughly 142 million kilometres away. Earth is the only planet that is in the habitable zone which is 150 million kilometres away from the sun. My point is that not even Mars is in the habitable zone and it is only around 225 million kilometres away from Earth. Europa is much, much farther. What is a stars habitable zone? It is the next best way for finding life, and it really narrows down where life would most likely be. A stars habitable zone has to do with water and Goldilocks. Water too close to a star will dry up, but water too far away will freeze like it has on Europa. However, water at a perfect distance from its star, is just right, (just like Goldilocks’ porridge) and it will remain a liquid. As I said before, water is super important and so, scientists have found a way to tell whether a planet is in their stars habitable zone using space telescopes like Kepler and TESS (Transiting Exoplanet Survey Satellite). Jamie Shreeve wrote “Like Kepler, TESS looks for a slight dimming in the luminosity of a star when a planet passes–transits–in front of it. TESS is scanning nearly the whole sky, with the goal of identifying about 50 exoplanets with rocky surfaces like Earth’s that could be investigated by more powerful telescopes coming on line.” With telescopes like these, we can identify what planets are in their stars habitable zone, but until scientists build a spacecraft that can reach these planets we don’t know exactly what is there, although we do know where to look once we’ve gotten there.
The last method that I found to narrow down where life would be is by using the chemical reaction of methane and oxygen. Jamie Shreeve writes “Oxygen is a flagrantly promiscuous molecule–it’ll react and bond with just about everything on a planet’s surface. So if we can find evidence of it accumulating in an atmosphere, it will raise some eyebrows. Even more telling would be a biosignature composed of oxygen and other compounds related to life on Earth. Most convincing of all would be to find oxygen along with methane, because those two gases from living organisms destroy each other. Finding them both would mean there must be constant replenishment.” So far, researchers have not found these two gases together, but like Jamie Shreeve said, if we find oxygen and methane together, there would be a high chance that its because of life.
To answer the question of where life would most likely be if we found it, I would say, find a planet that has water, that is in its stars habitable zone, and that has oxygen and another gas, particularly methane, existing together. If there is a planet out there with all of these conditions then it’s very possible some form of life exists on that planet. Even though the chances are that this is where life is, we can never be sure, and according to the article The search is on: new missions and discoveries on Earth within our solar system and beyond are bringing us closer than ever to finding alien life on other planets, “John Priscu is similarly open-minded about the appearance of alien life. “I bet we have looked it straight in the face already,” he says, “but didn’t know what we were looking for.””
Bibliography:
Abbasi, Jennifer. “The search is on: new missions and discoveries on Earth within our solar system and beyond are bringing us closer than ever to finding alien life on other planets.” Popular Science, Oct. 2011, p. 37+. Gale In Context: Science, https://link.gale.com/apps/doc/A267026292/GPS?u=43riss&sid=GPS&xid=c1eb2e9c. Accessed 9 Sept. 2019.
Barone, Jennifer. “Are we alone? A NASA telescope cranks up the search for alien planets.” Science World/Current Science, 6 May 2013, p. 14+. Gale In Context: Science, https://link.gale.com/apps/doc/A328850579/GPS?u=43riss&sid=GPS&xid=397a9362. Accessed 9 Sept. 2019.
Powell, Corey S. “Europa or bust.” Popular Science, Sept. 2015, p. 54+. Gale In Context: Science, https://link.gale.com/apps/doc/A426149579/GPS?u=43riss&sid=GPS&xid=03718137. Accessed 9 Sept. 2019.
Shreeve, Jamie. “WHO’S OUT THERE? NEW DISCOVERIES REVEAL IT’S ALMOST CERTAIN WE’RE NOT ALONE IN THE UNIVERSE. HERE’S HOW WE’RE SEARCHING FOR LIFE-AND TRYING TO MAKE CONTACT.” National Geographic, Mar. 2019, p. 42+. Gale In Context: Science, https://link.gale.com/apps/doc/A583382035/GPS?u=43riss&sid=GPS&xid=7acc4cd7. Accessed 9 Sept. 2019.
Picture by pennstatenews is licensed under CC BY-NC 2.0
Reflections