Introduction: In this lab, we will explore a method to determine the concentration and identity of a base using titration. The base solution is unknown, but we have access to a solid form of an acid called oxalic acid dihydrate [(COOH)2 x 2H2O]. By measuring the volume of acid required to neutralize a known volume of the base solution, we can calculate the concentration of the base. Additionally, we will perform solubility tests using various indicators to help identify the base.
Materials:
- 200 mL of oxalic acid dihydrate [(COOH)2 x 2H2O]
- Unknown base solution
- Pipette
- Burette
- 1 Erlenmeyer flask
- 2 Beakers (100 mL), one for the base and another for the acid
- Phenolphthalein indicator (pink)
- Phenolphthalein dropper
- Stand and clamp
- Spot plate
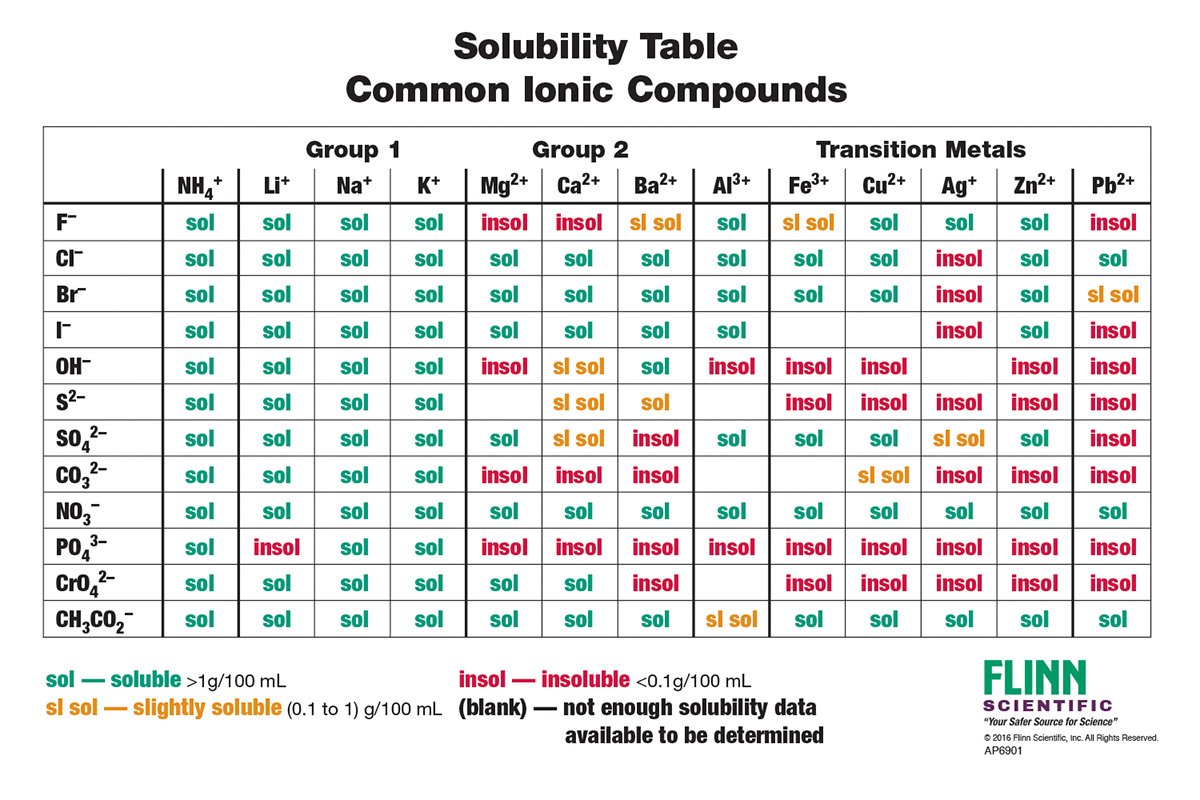
- Indicators: cobalt chloride, lead nitrate, sodium phosphate, potassium iodide, barium nitrate, aluminum sulfate, sodium sulfate (Na2SO4), and sodium sulfide (Na2S)
Procedure:
- Setting up the titration: a. Set up a dropper and a burette. Fill the burette with the unknown base solution and carefully roll it to ensure the inner surface is coated with the base. B. Dissolve the solid oxalic acid dihydrate in water and transfer 10 mL of the resulting solution into the Erlenmeyer flask. c. Add 3-4 drops of phenolphthalein indicator to the flask.
- Performing the titration: a. Begin adding the base solution drop by drop from the burette into the flask while swirling the flask gently. b. Observe the color change in the solution. Continue adding the base until the solution turns to the lightest possible pink. c. Immediately turn off the burette’s flow when the solution turns pink. d. Record the volume of base solution used to the nearest 0.01 mL.
- Calculation of base concentration: To determine the concentration of the base, we will use the stoichiometry of the reaction between the acid and the base. a. Calculate the moles of acid used in the titration: (volume of acid used) x (acid concentration). b. Use the balanced chemical equation to determine the moles of base used, which should be equal to the moles of acid used. c. Calculate the concentration of the base by dividing the moles of base used by the known volume of base solution.
- Solubility tests: a. Set up the spot plate and add a small amount of each indicator to separate spots on the plate. b. Add a few drops of the base solution to each spot containing an indicator. c. Observe and record any color changes or precipitate formation in each spot.
- Analysis of reactions: a. Research the expected reactions between the indicators and the base. b. Compare the observed reactions in the spot plate with the expected reactions to identify the base.
Given information.
Oxalic acid dihydrate (COOH)2 x 2H2O
Molar mass: 126g/mol
Base – 0-0.05
Titration
Molarity of the Base
Trail 1 Initial reading 0ml, Final reading 27.3ml
Trail 2 Initial reading 27.3ml, Final reading 8.3ml
rail 3 Initial reading 34ml, Final reading 2ml



average vol = 24.6ml
Solubility
| solution | CoCl2 | Pb(NO3)2 | Na3PO4 | KI | Ba(NO3)2 | Al2(SO4)3 | Na2SO4 | Na2S |
| unknown base | R (bluish green) | R (cloudy) | R (cloudy | nr | nr | nr | nr | nr |

Calculations:
(COOH)2 x 2H2O -> CaC2O4+2H2O
Acid moles to find base moles = 0.02M(C2H2O4*2H2O)= xmol/o.o1L = 0.0002molC2H2O4*2H2O
0.0002molC2H2O4*2H2O = 1molCa(OH)2/1molC2H2O4*2H2O
Base Volume=
24.6/1000ml = 0.0246L
Base concentration
M= 0.0002molCa(OH)2/0.0246L
0.01M Ca(OH)2
0.02MOH-
conclusion:
To identify the base, we initially hypothesized that it belonged to the alkali group. To test this, we conducted experiments using phosphate, which resulted in the formation of a precipitate. To narrow down the options, we performed a solubility experiment and found that the base was likely either Calcium Hydroxide or Barium Hydroxide. When we googled Calcium Hydroxide it says its slightly soluble in hydroxide solutions, resulting in a cloudy appearance. By observing the cloudiness of the solution, we concluded that our base formula was Ca(OH)2. Now, our next objective was to determine its concentration. To obtain the concentration of the base, we conducted a titration experiment, employing Phenolphthalein to detect the endpoint. By averaging the volume of acid used in three trials, we determined the moles of acid consumed. Since the acid and base have a 1:1 ratio, we assumed that the moles of base consumed were equivalent. Consequently, we calculated the concentration of the base using the volume and moles employed in the titration. It is important to acknowledge potential sources of error in our procedure. Despite our efforts to obtain accurate results by using a pH indicator, achieving precise coloration proved challenging. Even slight variations in the amount of base added during the titration could yield different concentration values for the unknown base. To mitigate this issue, one could consider increasing the quantity of pH indicator used to obtain more distinct colors. However, caution must be exercised to avoid excessive usage, as it may alter the pH of the solution. Alternatively, obtaining a pH indicator specifically calibrated for the acidity levels of the acids and bases employed in the reaction could provide more accurate results.
