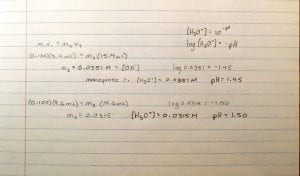Purpose: To find the pH and concentration of acid
Materials:
- 50 mL water
- 5.70g of nerds (Malonic Acid)
- 30 mL of sample
- phenolphthalein
- Stir stick
- Graduated cylinder
- 3 funnels
- 3 filer sheets
- Erlenmeyer flask
- 3 beakers
- Ring stand and mixer
- 15 mL of 0.10 M NaOH
Procedure:
- crush nerds into a fine powder. Measure mass and record.
2. Dissolve powdered nerds in 50 mL of water and mix with a stir stick.
3. Place filter sheets in the funnels and filter the acid solution.
4. Measure out 10 mL of the acid solution.
5. Add two drops of phenolphthalein to 10 mL of solution.
6. Titrate 10 mL of solution by adding 0.10 M NaOH.
7. Continue to add NaOH until ppt begins to turn pink, thus indicating the solution is neutral. Repeat 3 times.
8. Record how much of the basic solution was added.
Data:
| Trial # | Volume of Acid (mL) | Volume of NaOH (base) (mL) |
| Trial 1 | 10 mL | 5.40 mL |
| Trial 2 | 10 mL | 4.60 mL |
| Trial 3 | 10 mL | 4.60 mL |
| Average Volume | 4.87 mL |
Calculations:
since two of the trials had the same volume of NaOH used, we only wrote two equations.
Conclusion:
In conclusion, we found that acids are a recurring part of our daily lives. It was surprising to us that the candy had a fairly low amount of a strong acid. We found the concentration of our acid by diluting it in water and filtering it. We then used titration to help us find the pH of the candy. The phenolphthalein was an important aspect that informed us when our solution was neutral by turning pink. We used the M1V1 = M2V2 formula in order to find the concentration of hydronium ions, helping us find our final pH. Our final pH was between 1.45 and 2 which makes sense since the pOH of the base is about 13.