This article caught my eye because I am a big sports fan. I knew thru other news stories that football was the worst sport for head injuries and long term brain trauma, however what caught my eye was that soccer, which is my favourite sport, has the second highest rate for head injuries behind football, according to this article. I liked how the author expresses the information by using many facts and statistics to back up the story. This article reminded me of how when I used to play soccer, some of my teammates got concussions. I personally never got a concussion, however I know people who have had to quit due to getting serious concussions concussions.
Monthly Archives: March 2019
Blog log 1 – Internet as political media
This article interested me because it illustrates how the internet can be used as a tool for political reasons. The quality of this article was very good because the author remained objective and presented points from both sides. In the article, a man was sent to jail for a month for just a simple tweet, which made me think about how in our world right now, we do not have a lot of free speech. The article really made me think about our modern society and how almost everything, such as political issues, are using the internet as their primary media to achieve a particular political agenda. I also thought about how the internet makes it a lot harder to find meaningful news that you are 100 percent sure is unbiased and real, because with the internet there also comes a lot of fake news and it is hard, at times, to differentiate between the good and the bad.
Measuring Keq Chemistry Lab
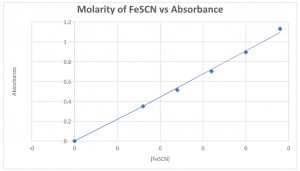
Part I: Preparation of a standard absorption curve for FeSCN+2
| Standard | 0.20M Fe(NO3)3 | 0.0020 M KSCN | 0.100M HNO3 | [FeSCN+2] | Absorbance |
| A
|
10.0 mL | 0.0 mL | 15.0 mL | 0 | 0 |
| B
|
10.0 mL | 1.0 mL | 14.0 mL | 0.00008 | 0.350 |
| C
|
10.0 mL | 1.5 mL | 13.5 mL | 0.00012 | 0.514 |
| D
|
10.0 mL | 2.0 mL | 13.0 mL | 0.00016 | 0.702 |
| E
|
10.0 mL | 2.5 mL | 12.5 mL | 0.00020 | 0.895 |
| F
|
10.0 mL | 3.0 mL | 12.0 mL | 0.00024 | 1.130 |
EQUATION: y=4529x R2__0.9957_____
Part 2: Measuring Equilibrium
| Test Solution | 0.0020 M Fe(NO3)3 | 0.0020 M
KSCN |
0.10 M
HNO3 |
Initial [Fe+3] | Initial [SCN–] | Absorbance | Equilibrium
[FeSCN+2]* |
| I
|
5.0 mL | 0 | 5.0 mL | 0.001 | 0 | 0 | 0 |
| II
|
5.0 mL | 1.0 mL | 4.0 mL | 0.001 | 0.0002 | 0.162 | 0.000036 |
| III
|
5.0 mL | 2.0 mL | 3.0 mL | 0.001 | 0.0004 | 0331 | 0.000073 |
| IV
|
5.0 mL | 3.0 mL | 2.0 mL | 0.001 | 0.0006 | 0.508 | 0.000112 |
| V
|
5.0 mL | 4.0 mL | 1.0 mL | 0.001 | 0.0008 | 0.612 | 0.000135 |
| VI
|
5.0 mL | 5.0 mL | 0.0 mL | 0.001 | 0.0010 | 0.796 | 0.000176 |
* To be determined from the standard graph equation.
ANALYSIS:
- Use your graph equation to calculate the equilibrium concentrations of FeSCN+2.
- Prepare and ICE chart for each test solution (II – VI) and calculate the value of Keq for each of your 5 tests solutions.
ICE CHART II
| Test Solution
Keq =227.7 |
Fe3+ + SCN– ⇄ FeSCN2+ | ||
| I
|
0.001 | 0.0002 | 0 |
| C
|
-0.000036 | -0.000036 | 0.000036 |
| E
|
0.000964 | 0.000164 | 0.000036 |
ICE CHART III
| Test Solution
Keq =240.8 |
Fe3+ + SCN– ⇄ FeSCN2+ | ||
| I
|
0.001 | 0.0004 | 0 |
| C
|
-0.000073 | -0.000073 | 0.000073 |
| E
|
0.000927 | 0.000327 | 0.000073 |
ICE CHART IV
| Test Solution
Keq =258.5 |
Fe3+ + SCN– ⇄ FeSCN2+ | ||
| I
|
0.001 | 0.0006 | 0 |
| C
|
-0.000112 | -0.000112 | 0.000112 |
| E
|
0.000888 | 0.000488 | 0.000112 |
ICE CHART V
| Test Solution
Keq =234.7 |
Fe3+ + SCN– ⇄ FeSCN2+ | ||
| I
|
0.001 | 0.0008 | 0 |
| C
|
-0.000135 | -0.000135 | 0.000135 |
| E
|
0.000865 | 0.000665 | 0.000135 |
ICE CHART VI
| Test Solution
Keq =259.2 |
Fe3+ + SCN– ⇄ FeSCN2+ | ||
| I
|
0.001 | 0.001 | 0 |
| C
|
-0.000176 | -0.000176 | 0.000176 |
| E
|
0.000824 | 0.000824 | 0.000176 |
CONCLUSION AND EVALUATION:
- Comment on your Keq values. Do your results convince you that Keq is a constant value regardless of the initial concentrations of the reactants? Why or why not?
Yes. Although there is some difference in the Keq, they are close enough to assume that equilibrium concentration does not affect the Keq.
- Calculate the average value of Keq from your five trials. The actual value of Keq for this reaction at 25oC is reported as 280. Calculate (should you use all of your values?) the percent difference of your average value from the reported value:
Avg Keq=244.2
% difference = (|experimental value – reported value|) x 100%
Reported value
% difference = (|244.2-280|) x 100 = 12.8%
280