DEFINE
- The science community has offered you to try to make a new periodic table of our own. The task at hand is about solution fluency and collaboration fluency.
- We have been requested to make a new periodic table and customize it to our own.
- We need to make the new periodic table more ‘hip’ so the youths of this country will be more interested and be more involved.
FINAL STATEMENT: The science community has offered us to try and establish a periodic table of our own. Are we capable of constructing a new and improved periodic table? The task at hand is about the progression of solution and collaboration fluency that was discussed in class with Mr. Robinson.
DISCOVER
- How are we going to separate the elements, for example by atomic number? number of protons etc.
- How can we use various colours and shapes to isolate and order the elements?
- Which shape will be most efficient for all the information to fit into?
- Do we have to include every element of the original periodic table?
- Should we make the new periodic table 3D, digital, or on paper form?
- Should we group and organize the elements by the family groups?
- Does anyone know any apps that can help us do this assignment?
- What are each of our talents and how can we use it to improve our periodic table?
- Should we consider ordering the elements by their physical or chemical properties?
FINAL STATEMENT: (the questions above)
DREAM
- We need to add and keep all of the atomic #, atomic mass, ion charges, symbol, and the name. We could arrange it differently by ordering the elements by number protons or electrons for example one row being all elements with seventy protons or under. We could use a sphere because it gives us more freedom in how we can arrange the information.
FINAL STATEMENT: We can utilize spheres to give us more freedom on how we arrange the information. We desire to keep the atomic number, atomic mass, ion charges, the symbol, and the name on the sphere. The categories can be spread between the families or the periods. Our periodic table will be the same as the original, it will just be in sphere form.
DESIGN
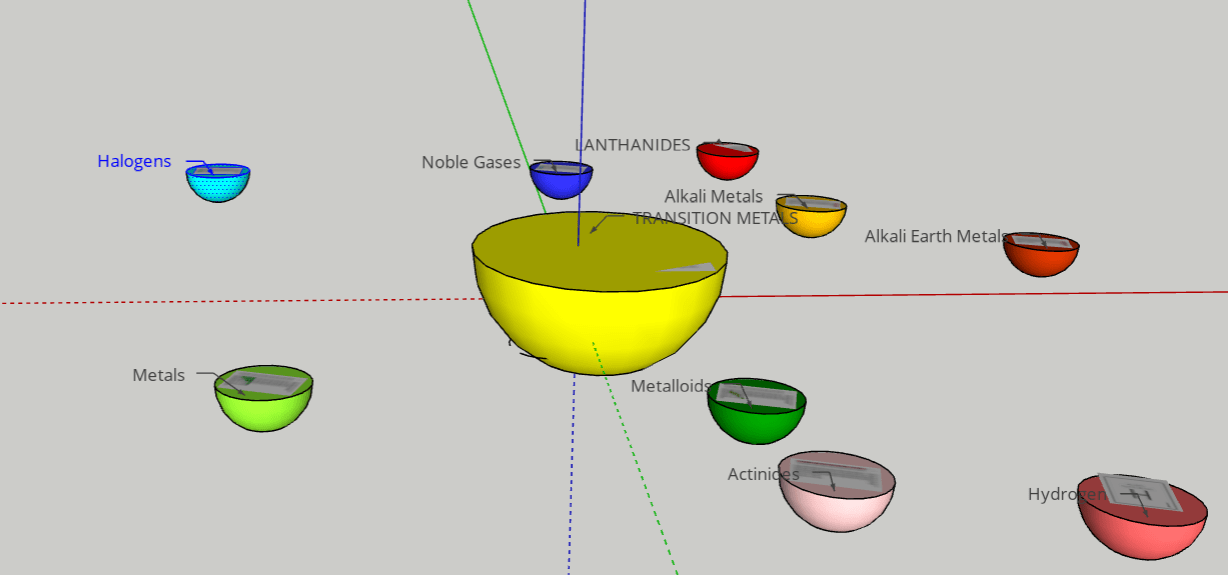
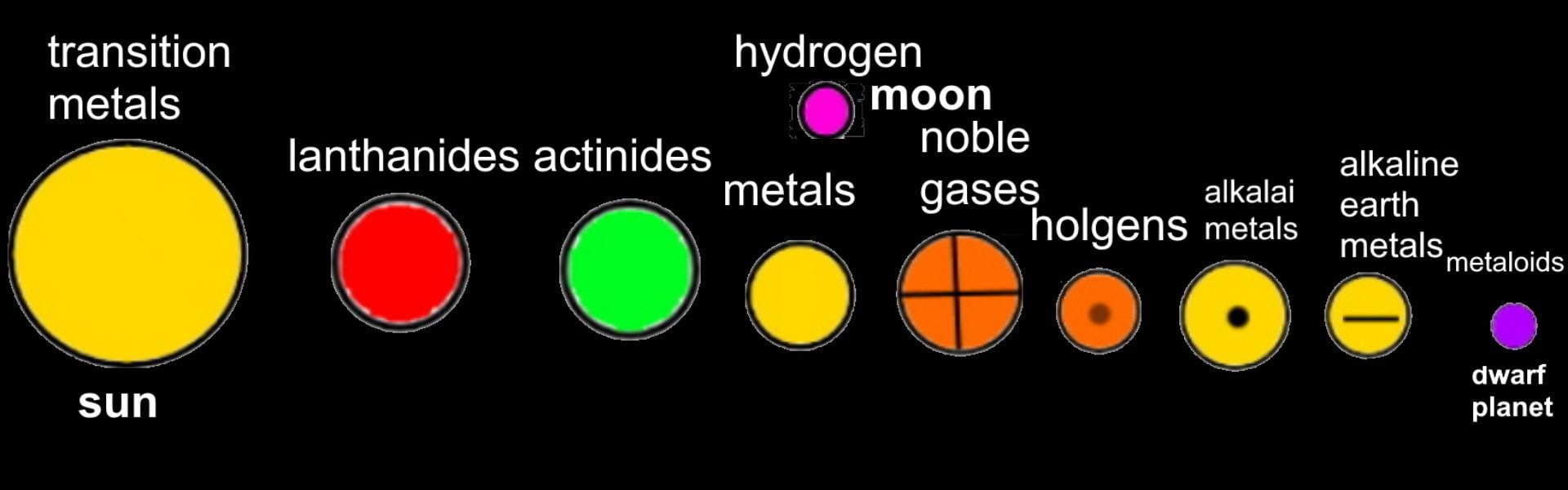
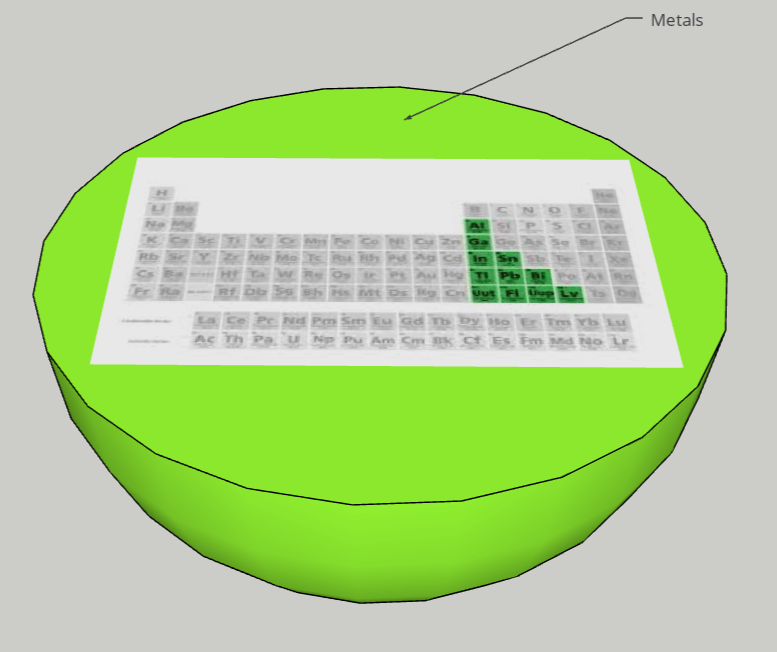
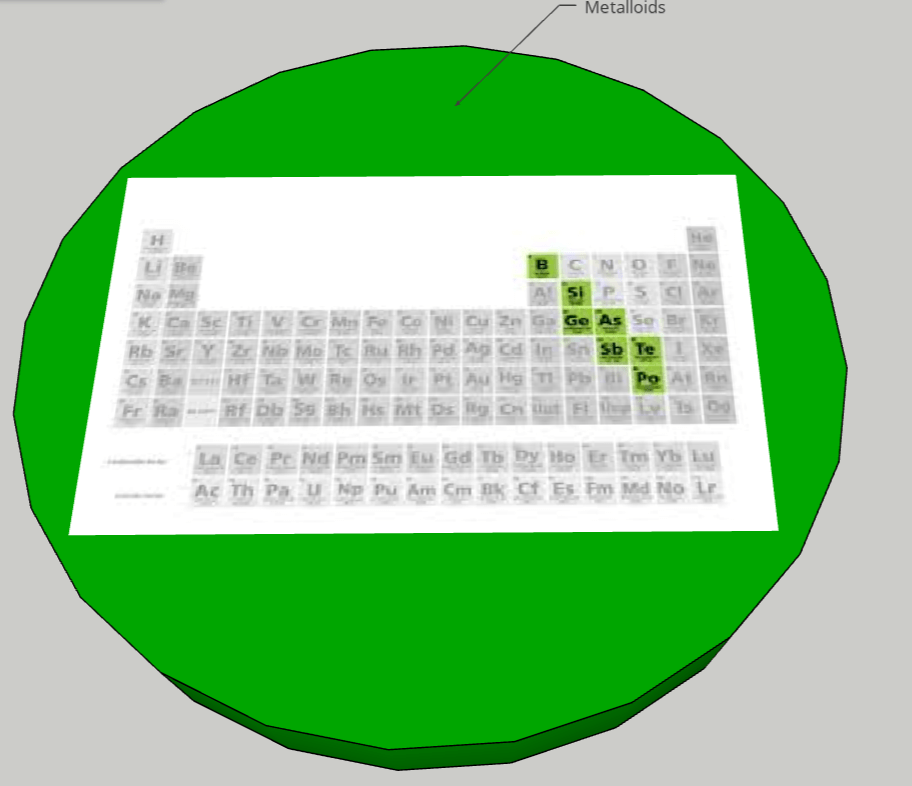
FINAL STATEMENT: We desire to assemble a solar system with planets that obtain the elements inside of them. The planets can open up into quarters and it will have layers that have the information of each element. The different planets will be the categories of the element families (alkali metals, halogens, noble gases, etc.). Hydrogen will be the moon (apart from the other planets). We will base the size of each planet by the number of elements there are in each category. We will try to have names for each planet for more creativity and for labels. We will use the sun for transition metals because it has the greatest number of elements in it and dwarf planet for Metalloids because it has the least number of elements. We think that grouping the elements by its families was the best choice for our plan because we wanted a decent number of planets to make a solar system.
DELIVER
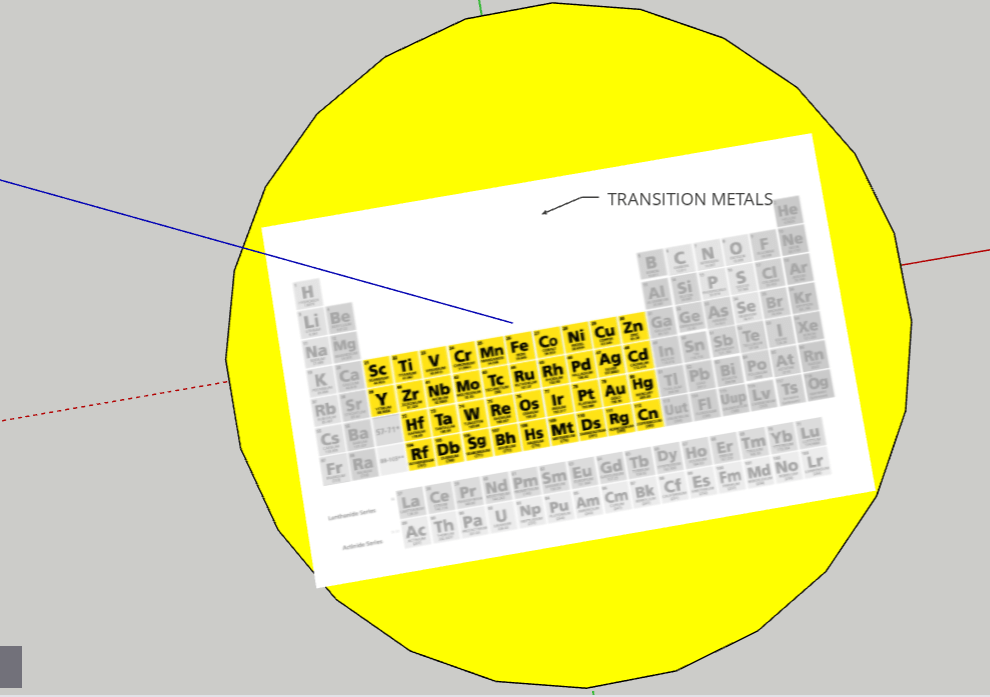
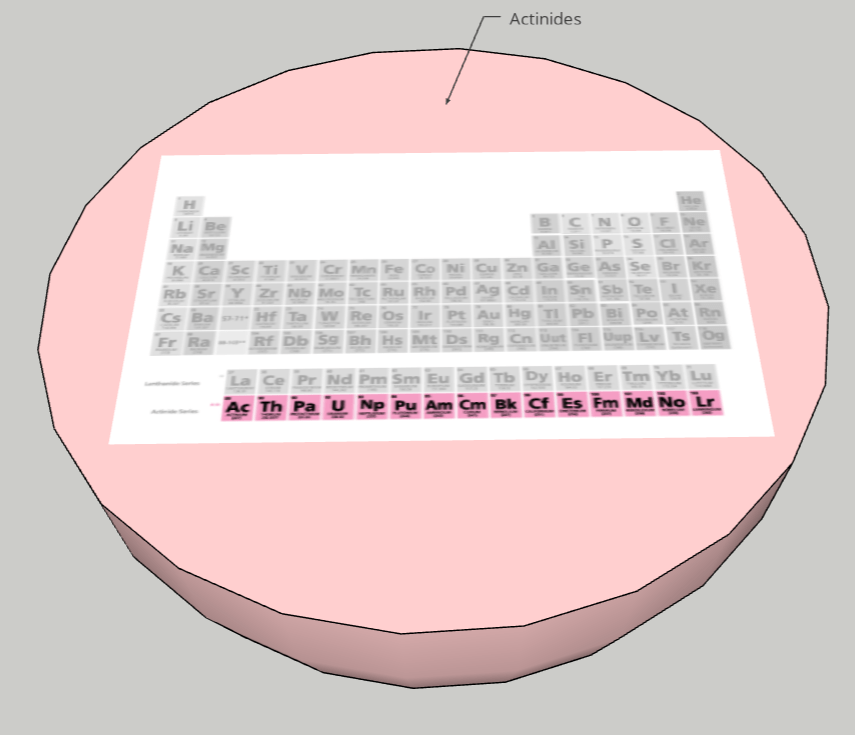
FINAL STATEMENT: Our periodic table is a model of the solar system. Firstly, the sun is a star for the transition metals. We chose the sun because it has the greatest number of elements in it. Next, we have the twin planets who are planets representing the Lanthanides and Actinides. They had the same number of elements, so we made it into twin planets. For the next one, we have the metals. Then, the noble gases. We also have a dwarf planet for the Metalloids. The Metalloids are in a dwarf planet because it has the least number of elements in it. The next three planets are Halogens, Alkali Metals, and Alkaline Earth Metals. Our final one is the moon, which represents Hydrogen. We decided to isolate Hydrogen by itself because it is the first element on the table. Each planet (category) will be opened in half so that everything inside can be seen. There are pictures of the elements applicable for the category. Each element has the information of the atomic number, the mass, ion charges, the symbol, and the name. We didn’t add any names because with the names, we felt that it could’ve been confusing to find the group of elements that you were looking for. We decided to keep the labels as the family group the elements were in.
DEBRIEF
FINAL STATEMENT: We revised back on the process it took us to come up with a plan, and we were proud at how we really worked as a group and made the most of each of our capabilities and valuable skills. We felt that our periodic table was unique. All three of us combined our ideas together and came up with an exclusive plan for our final result. As a group, we could’ve improved on starting the design process sooner. For us, the design process took the most amount of time and because we had a due date, we tried our hardest to make the planets at the end look nice but have the necessary information. A road bump we had along the way was that we had to regain the memory and think back on how to use google sketch up. It’s been a couple years, so we forgot how to use it, but we searched some instructions and remembered how to use it. We chose google sketch up because most of us learned how to use it before and we just had to think back on how to add pictures and create spheres. At the end, we decided on the planet because when we combined all of our ideas together, that was the obvious choice on the best outcome.







One Response