A. Atomic radius versus atomic number
A number of physical and chemical properties are related to the sizes of the atoms, but atomic size is somewhat difficult to define. There is no precise outer boundary of an atom. The radius is one half the distance between the centers of two adjacent atoms. The radius of an atom depends on the environment in which it is found. For bonded atoms, we customarily speak of a covalent radius, ionic radius, and, in the case of metals, a metallic radius. For atoms that are not bonded together, the radius is known as the van der Waals radius. For comparison, all radii in the above table are covalent.
1. Which is the largest of the first 54 elements?
Rubidium
2. Describe how the atomic radius varies within a period and within a family.
An atom gets larger as the number of electronic shells increase, the radius of atoms increases as you go down a family. The size of an atom will decrease as you move from left to the right in a period.
3. Use your graph to predict the atomic radius of the following elements:
a. cesium = 0.223
(b) tungsten = 0.142
(c) thallium = 0.166
(d) radon = 0.143
4. Which group of the main group elements contains the largest elements?
Alkali Metals
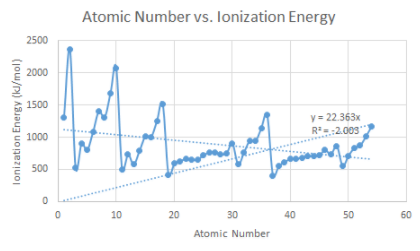
B. Ionization energy versus atomic number
5. How would you explain ionization energy to your partner?
b.How does the ionization energy vary within a period and within a family?
Elements on the right side of the periodic table have a higher ionization energy because their valence shell is almost full.
c. Which element on your graph has the strongest hold of its valence electrons?
He Helium
6. (a) Write the electron configuration for chlorine.
[Ne] 3s2 3p5
(b) Which electron is lost when 1251 kJ/mol of energy are applied to a sample of chlorine atoms?
2. Compare the ionization energies of metals to nonmetals
Metals have lower ionization energies and nonmetals have higher ionization energies
C. Melting point versus atomic number
7. Describe the trend of melting points within a period
There is not really a specific trend for melting points along the periods, however metals tend to have a higher
melting point and non-metals tend to have a lower melting point.
8. Which group of elements tends to have the highest melting points
Carbon and Boron have the highest melting points within the elements with atomic numbers 1-54.The transition
metals tend to have the highest melting points.
9. Tungsten is used in incandescent light bulbs because it has an extremely high melting point. Which element on your chart could be
a reasonable replacement for tungsten? Why?
Carbon could be a reasonable replacement for Tungsten, since Carbon has a melting point of 3,550 and
Tungsten has a melting point of 3,422.
D. Density versus atomic number
10. Describe how density varies within a period.
Usually, density increases when you go from left to right and top to bottom. Gaseous substances will be substantially lower than solids and liquids.
Compare the densities of the elements in the second period with the elements in the third period.
The density increases as you go from left to right so the density will increase as you go from second to third period.
Assume that the transition metals given in the table are representative of the other members of this group. How do the densities of the transition metals
compare with those of the elements in the main group?
Transition metals have hard and high densities and elements in the main group have light and soft densities.
Explain why aluminum and magnesium are more suitable than iron for use in some airplane parts.
Aluminum and magnesium are light and durable whereas iron is dense and heavy.
E. Electronegativity versus atomic number
11. Describe how electronegativity varies within a period.
The electronegativity will increase as the number of protons in the nucleus increase
12. Describe how electronegativity varies within a family.
As the energy levels increase, the electronegativity decreases since it gets further away from the nucleus