Matter Matters – ADL 10
Define
What is Matter?
A physical substance in general, as distinct from mind and spirit. It is also that of which occupies space and possesses rest mass, especially as distinct from energy. The examples below are all under the title matter because they all take up space.
Qualitative properties are properties that are observed and can generally not be measured with a numerical result. They are contrasted to quantitative properties which have numerical characteristics.
A pure substance is a material that is composed of only one type of particle; examples of a pure substance include gold, oxygen and water. A mixture is a material made up of at least two different pure substances. Mechanical mixture is a mixture in which each material maintains its own properties.
Chemical Change: Burning, rusting, and cooking are all examples of chemical change. In this example, cooking is used for chemical change. When you are making an egg, first you have a runny liquid spill on to the pan, then once it heats up a bit, it starts to change to a solid.

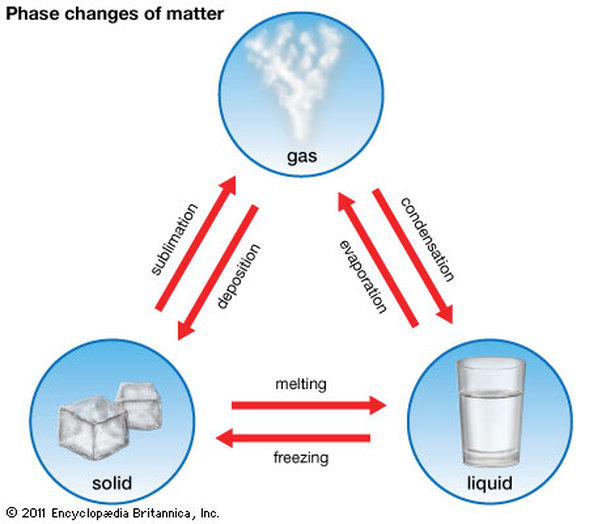
Changes of State: The changes of state include being a solid, liquid or a gas. Water is the example used below which is one simple material that can be all three. The changes of state all depend on the weathering and temperature of the substance. For example when water heats up, it evaporates into a gas. To get to a solid, the gas has to go through the state of deposition. One example of deposition is the process by which, in sub-freezing air, water vapor changes directly to ice without first becoming a liquid. Now, all the water solid (ice) has to do is melt to become back to a liquid. These changes could also happen reversibly.
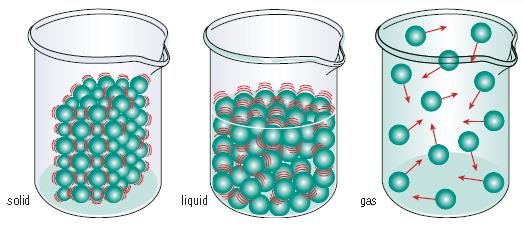
Kinetic Molecular Theory: The attraction of particles decreases with an increase in distance. Solids are tightly packed together particles that have very little or no space between them that only vibrate. Liquids don’t have much space between the particles and they fit according to the space it is being kept in. These have little amount of energy and can move around freely throughout the container. In a gas, the particles have lots of energy and move around very freely. These particles travel throughout the whole container by expanding and compressing to fill any size container.
Dream
Some of the ways I could display the information:
-Video
-Pictures and Explanations
-Document
-PowerPoint
Debrief
How did the process of completing this challenge go?
It went pretty well. The challenge was fun and quick. When I was researching I knew just what I was looking for.
What did you do well?
I was efficient and got it done quick. I learned some of this information from past years so I used my prior knowledge for examples.
What could you have done better?
More interesting ways to display the information of the project.

