Match and Water Trick – How does the water react with a burning candle?
Magic!
The water appears to magically rise upwards when the flame is extinguished. How does science explain this mystery?
Materials & Procedures
Materials: dish, candle, beaker, water, lighter
- Fill a shallow dish with some water and place a candle in the middle.
- Light the candle.
- Quickly place a glass beaker on top.
- Observe!
Chemical Reaction:
CH4 + O2 → CO2 + H2O (Complete combustion)
Research:
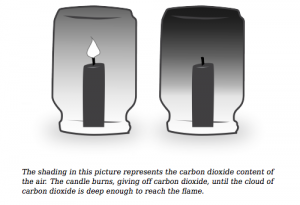
The beaker is placed over the candle, and the flame heats the air up. When the air is heated, the gas particles expand and move faster, putting pressure on the water (higher temperature = higher air pressure; Gay-Lussac’s law – pressure increases proportionately to temperature increase). Carbon dioxide, a product from the chemical reaction (CH4 + O2 → CO2 + H2O) pushes oxygen downwards since it is hotter (less dense). As more carbon dioxide is produced there is less oxygen available for the combustion, causing the flame to go out. This lowers the temperature of the air. Applying the Gay-Lussac Law, there is now less pressure on the water. The decrease in air pressure causes the water to rise.
A common explanation and misconception is how the oxygen burnt during combustion causes the water to rise; oxygen in the air is burnt, leaving an empty space, and the water rises up to fill it. This suggests that the volume of water risen is the same amount of oxygen used in the combustion. However, the conservation of mass tells us that the mass after the chemical reaction is the same as before because no atoms can be created or destroyed. In this case, the oxygen became the products, CO2 + H2O, and wasn’t destroyed.
Observations & Outcomes:
For our first attempt at this experiment, we used a 1L beaker to place over the candle flame. We soon realized that the flame will take too long to extinguish because there is too much oxygen, so instead we switched to a 250mL beaker which helped shorten the time. The flame extinguished and the water started slowly rising. The water stayed up for several minutes.
Sources:
Combustion of Hydrocarbons. (n.d.). Retrieved October 27, 2015, from http://www.ausetute.com.au/combusta.html
Knill, O. (2006, September 24). The burning candle – rising water experiment. Retrieved October 27, 2015, from http://www.math.harvard.edu/~knill/pedagogy/waterexperiment/
Rudel, D. (n.d.). Chapter 1 of Volume 2: Candles Unders Jars. Retrieved October 27, 2015, from http://misconceptions.science-book.net/free-samples/
The Naked Scientists. (n.d.). Retrieved October 27, 2015, from http://www.thenakedscientists.com/HTML/experiments/exp/losing-air/
Document link: https://docs.google.com/document/d/1122u0bRJDFUBANOtCbFBIat51ZDsxzAM6Fu1PnGThg8/edit?usp=sharing
Video: https://www.dropbox.com/s/8xvhglonrlg3m07/Science%20is%20magic!!.mp4?dl=0
Link to partner’s blogpost: http://myriverside.sd43.bc.ca/hanaw-2014/2015/11/02/science-is-magic/