Matter Matters
Matter Matters
These concepts might be hard to understand because it uses words that aren’t spoken on a daily basis so people don’t recognize them. All these ideas fit into matter because all things that we can see and touch are matter. I would want to make a description of each area with a picture to help people understand what it means in a visual way.
Chemical Change
Chemical change is any change that results in the formation of new chemical substances. For example atoms from one element rearrange themselves into a different new element
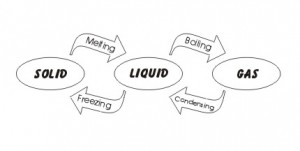
Change of State
A Change of state is when a liquid turns into a gas such as boiling water and the water evaporating. There are other changes of state as seen in the picture below
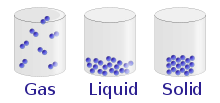
Particle Model of Matter
The particle model can be used to explain the properties of solids, liquids, and gases. It can also be used to explain what happens in changes of state. The particles of matter are always moving.
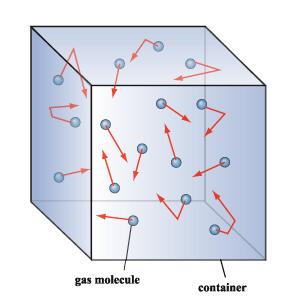
Kinetic Molecular Theory
The kinetic molecular theory shows how individual gas particles interact with one another. The kinetic molecular theory contains a number of statements compatible with the assumptions of the ideal gas law.
Qualitative and Quantitative Properties
Qualitative observations use your senses to observe the results. (Sight, smell, touch, taste and
sound.) Quantitative observations are made with instruments such as rulers, balances, graduated cylinders, beakers, and thermometers. These results are measurable
Pure Substances
A material that is composed of only one type of particle; examples of a pure substance include gold, oxygen and water. A material made up of at least two different pure substances. A mixture in which each material maintains its own properties.
I think I could have made it more engaging, but it is good for anyone who is a visual learner.