Chemical Periodicity
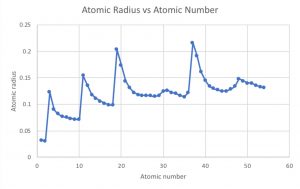
Atomic radius versus atomic number
A number of physical and chemical properties are related to the sizes of the atoms, but atomic size is somewhat difficult to define. There is no precise outer boundary of an atom. The radius is one half the distance between the centers of two adjacent atoms. The radius of an atom depends on the environment in which it is found. For bonded atoms, we customarily speak of a covalent radius, ionic radius, and, in the case of metals, a metallic radius. For atoms that are not bonded together, the radius is known as the van der Waals radius. For comparison, all radii in the above table are covalent.
1. Which is the largest of the first 54 elements? The largest of the first 54 elements is Rubidium (37) with an atomic radius of 0.216 nm
2. Describe how the atomic radius varies within a period and within a family. As you move left to right across a period the atomic radius tends to decrease, however, the atomic radius will increase moving top to bottom through a family
3. Use your graph to predict the atomic radius of the following elements:
(a) cesium: 0.280 nm (actual 0.267) (b) tungsten: 0.150 nm (actual 0.142) (c) thallium: 0.165 nm (actual 171) (d) radon: 0.135 nm (actual 0.141)
4. Which group of the main group elements contains the largest elements? The group of elements containing the largest elements are the Alkali metals located on the far left side of the periodic table.
Ionization energy versus atomic number 1. How would you explain ionization energy to your partner? The ionization energy is the amount of energy required to remove the most loosely bound electron, from the valence shell of its atom. Depending on the size of the atomic radius, there will be a stronger or weaker ionization energy binding that atom together.
2. How does the ionization energy vary within a period and within a family? Within a period moving from left to right the ionization energy increases due to smaller atomic radii, in a family moving from top to bottom, the ionization energy decreases due to larger atomic radii
3. Which element on your graph has the strongest hold of its valence electrons? Helium has the strongest hold of its valence electrons, not only is it a noble gas (on the far right of the periodic table) but it is also located in the top row of its family, this demonstrates that it has a very high ionization energy
(a) Write the electron configuration for chlorine. Chlorine: 1s²2s22p63s23p5
(b) Which electron is lost when 1251 kJ/mol of energy are applied to a sample of chlorine atoms? Because of chlorine’s natural stable state the chlorine atom would not likely gain nor lose an electron, however, a chlorine ion has a charge of -1 indicating that its outer P orbital (usually P5) would gain one electron to complete its valence shell thus changing it to P6
4. Compare the ionization energies of metals to nonmetals. Metals generally tend to have a higher ionization energy as they are located towards the right and top of the periodic table. Nonmetals typically carry a lower ionization energy and are found towards the bottom left of the table.
Melting point versus atomic number
1. Describe the trend of melting points within a period
Melting points vary for each period, they have patterns that depend on each of their positions in the periodic table. The melting points of group 1 elements decrease going down the group. This is caused by a decrease in the forces of attractions within the atoms.
2. Which group of elements tends to have the highest melting points
The group of elements that tend to have the highest melting point is group 14 because the elements with lower periods have similar melting points.
3. Tungsten is used in incandescent light bulbs because it has an extremely high melting point. Which element on your chart could be a reasonable replacement for tungsten? Why?
4. Tungsten has a high melting point and another element that could replace tungsten is platinum, it has a high melting point and can withstand heat.
Density versus atomic number
1. Describe how density varies within a period.
The first few periods with lower atomic numbers have a smaller mass than the periods with a higher atomic number.
2. Compare the densities of the elements in the second period with the elements in the third period.
The densities in the the second period are significantly smaller than the densities of the second period.
3. Assume that the transition metals given in the table are representative of the other members of this group. How do the densities of the transition metals compare with those of the elements in the main group?
The transition metals are very spread apart from each other compared to the rest of the group.
4. Explain why aluminum and magnesium are more suitable than iron for use in some airplane parts.
They are more suitable because they are lighter than iron would be on the plane.
Electronegativity versus atomic number
1. Describe how electronegativity varies within a period.
as you move across a period the electronegativity increases
Describe how electronegativity varies within a family.
as you move down a family the electronegativity decreases
References:
Kimball, D., E. Kuzub and M. Sanader (1993), Chemistry Laboratory Manual 1, Student’s Edition, Don Mills: Addison-Wesley Publishers Limited
Petrucci, R.H. (1982), General Chemistry, Principles and Modern Applications, 3rd ed. New York: Macmillan Publishing Co., Inc. Whitman, R.L., E.E. Zinck and R.A. Nalepa (1988), Chemistry Today 1, 3rd ed. Scarborough: Prentice-Hall Canada Inc.
Whitman, R.L., E.E. Zinck and R.A. Nalepa (1982), Chemistry Today Laboratory Manual, 2nd ed. Scarborough: Prentice-Hall Canada Inc.